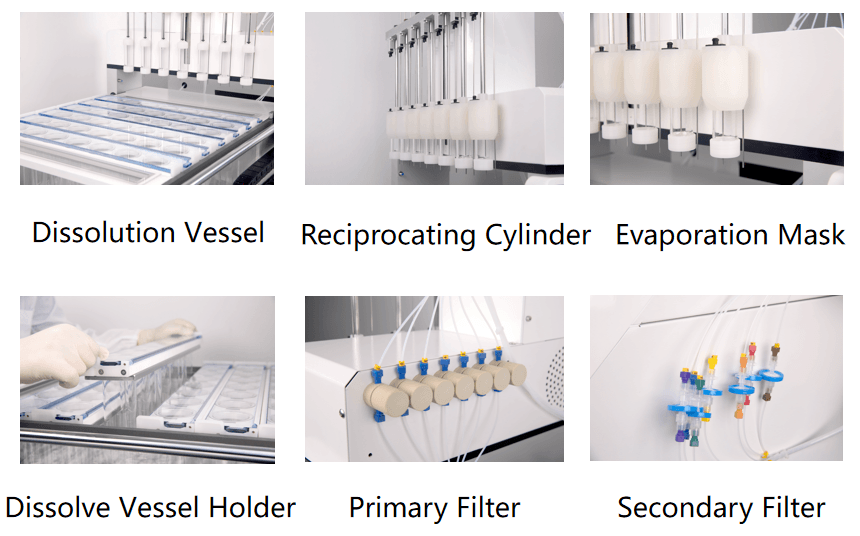
DS-BIOAT Reciprocating Cylinder
Product Introduction:
The DS-BIOAT automatic reciprocating cylinder dissolution system consists of a reciprocating cylinder dissolution device and an automatic sampling workstation. Meet the relevant requirements of the US Pharmacopoeia, Chinese Pharmacopoeia and European Pharmacopoeia. The device adopts a transparent hollow cylinder equipped with screens at both ends. The sample to be tested is placed in the cylinder and the screens at both ends are tightened before being placed in a dissolution cup containing dissolution medium and gently reciprocating up and down. The dissolution medium flows through a reciprocating cylinder through a sieve, providing shear force at the liquid-solid interface during the dissolution process of the drug. Suitable for the dissolution study of new drug formulations such as sustained-release, targeted release, and enteric coated delayed release formulations.
Main Features:
1. 300ml glass dissolution cup, 7-position, 6-row, in accordance with pharmacopoeia requirements.
2. The connecting rod type reciprocating structure ensures that the reciprocating stroke is fixed and unchanged.
3. Better leakage conditions have better dissolution effects for low solubility drugs.
4. The standard design meets the requirement of fully automatic switching between multiple media, better simulating changes in human pH environment.
5. Less media required, easy to change pH and good correlation with bioavailability data.
6. Online filtration function: two-stage filtration, columnar filtration, and 0.45um membrane circulation filtration. The 0.45um membrane is installed in the circulation pipeline to fully solve the problem of sample adsorption. During the experiment, no air is allowed to pass through the membrane, ensuring stable system pressure.
7. software system
① 8.4 inch touch screen control, Wahyong diffusion workstation, human-computer interaction, simple operation.
② Can configure 1000 accounts and 1000 experimental methods.
③ Paperless report management, capable of connecting to network printers, data can be stored locally and backed up to USB flash drives or enterprise servers.
④ Data audit tracking function, three-level permission management, in compliance with FDA 21 CFR Part 11 requirements.